Pulmonary Hypertension - Tectonic
NO LONGER ACCEPTING APPLICATIONS
Published on:
April 7, 2025
Sign-up Expiration:
Updated:
Locations:
McDonough, GA
Fort Mill, SC
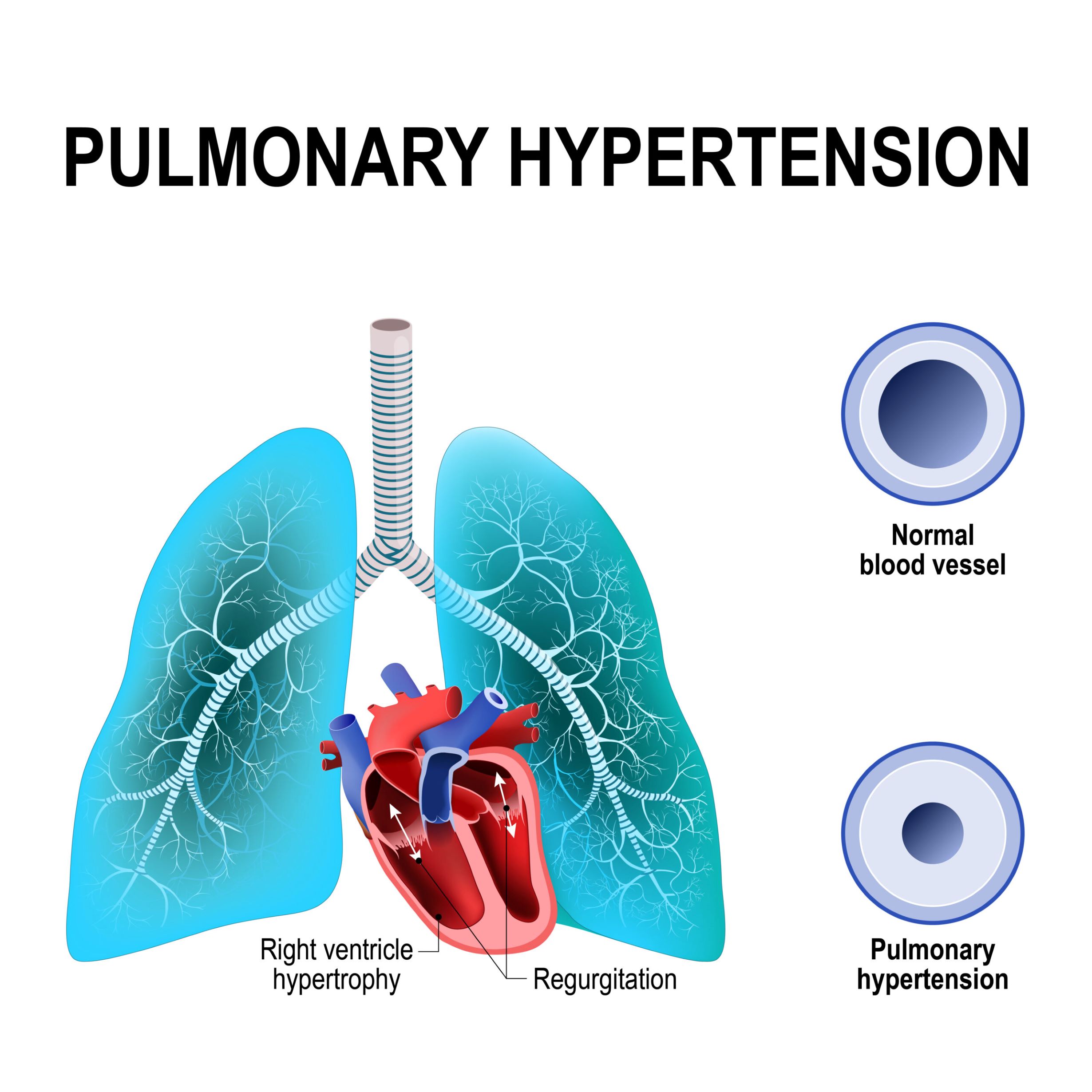
The primary objective of this study is to assess efficacy and safety of TX000045 after 24 weeks of treatment in patients with pulmonary hypertension secondary to heart failure with preserved ejection fraction (PH-HFpEF). There are currently no therapies approved for patients with PH-HFpEF. There is a need for a therapy that will reduce pulmonary hypertenstion (PH) and pulmonary vascular resistance (PVR) and improve left ventricular hemodynamics to treat this condition. TX000045 is a long-acting relaxin memetic that his demonstrated relevant hemodynamic effects in chronic models of PH and in heart failure.
ENDED







